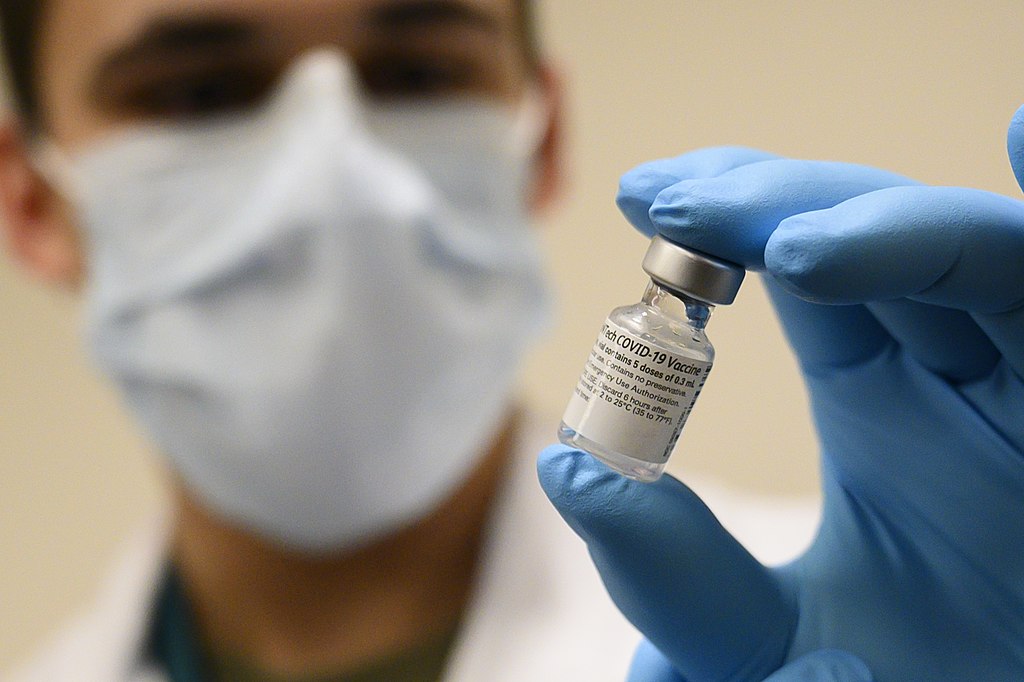
The vaccination programme is finally being rolled out, which can only be good news. Here in Hastings, we were off to a slow start, with the public vaccination programme not getting underway till January 4th. But although we’re by no means the world’s fastest nation at getting the vaccination into recipients, it’s good to see the UK, for once, pretty quick off the mark. But is the decision to give only one dose and delay the booster dose the right thing to do? And with this ’one dose’ strategy, can we guarantee anywhere near the two million vaccinations a week that the government has promised?
The decision to give just one dose of the vaccine isn’t based on any evidence from the vaccine trials. For the Pfizer vaccine, everyone in the trial was given a second dose after 21 days, so there’s no evidence at all from the trials that the vaccine works on one dose. The only evidence from the Oxford vaccine trials is based on mistakes made in the trials, where participants were given half doses of the vaccine, or the second dose was delayed. Those results appear to indicate that delaying the second dose by up to 12 weeks doesn’t affect the overall efficacy of the vaccine, but there are arguably too few results to be meaningful. So why take the risk?
The decision to give only one jab is based on two things. Firstly, past experience, where other vaccine programmes have shown similar efficacy of vaccines when the second dose has been administered anywhere between three and 12 weeks after the first. As most vaccines work in much the same way, it’s a reasonable assumption that the same will be true for the Oxford and Pfizer vaccines. Indeed, the WHO has now said that they have no objection to a delayed second dose of the Pfizer vaccine, as they believe it won’t affect the overall efficacy. Secondly, it’s a decision based on the protection given to society as a whole, rather than the individual. If you delay the second jab, you can give twice as many people their first jab within that initial twelve-week period. So if the vaccine is 90% efficacious, and you have ten million doses, if you give everyone two doses it’s likely that you can protect 4.5 million people. If you give only one dose, the percentage efficacy might be lower. But unless it’s lower than 45% efficacious with just a single dose, you’re going to be able to protect more people. If it drops to, say, 60%, you’ll still be protecting 6 million people. So while each individual person protected will get lesser protection than if they had the second dose, the number of people protected will be greater. Those percentages might not be quite true, based on the definition of percentage efficacy that I explain below, but it illustrates the overall impact.
Is this based on scientific evidence? Yes, if you look at data based on long-term use of other vaccines. No, if you look at data specifically from the trials of the Pfizer, Moderna and Oxford vaccines. We all know our Prime Minister is a chancer, so it doesn’t fill me with confidence when he takes a chance like this. Having said that, this is probably the right thing to do. Probably. But it’s not a gamble any other country seems to be taking, so far.
It’s worth reflecting perhaps on the nature of these vaccine trials, and what we mean by ‘efficacy’ of the vaccine. When we talk about a vaccine being 90% efficacious, it means that the number of people in the vaccinated group in the trial who got the disease was only ten percent of the number in the control group (who received a placebo). So it doesn’t strictly mean that a 90% efficacious vaccine will protect 90% of those vaccinated from the disease. I was surprised to discover that there’s no standardised, WHO-approved methodology for conducting vaccine trials. The trials are structured by the vaccine developer, with the methodology designed to satisfy national state regulators in the countries where they intend to market the vaccine. There isn’t even a standard definition of ‘efficacy’. Does it mean protection from any disease symptoms, lack of positive tests for the virus in the vaccinated and control groups, or prevention from the ‘full blown’ disease symptoms? This definition of efficacy is made for each individual trial. For the Covid vaccines, there were differences between the methodology of the different vaccine trials, so it is difficult to make direct comparisons between them, particularly in terms of ‘efficacy’. Indeed, from the descriptions I’ve looked at, none of the trials tested all the participants routinely for presence of the Covid-19 virus. At best, they only tested individuals who displayed symptoms. Overall, it seems that the Pfizer vaccine offers better protection against getting the virus and showing symptoms, and the Oxford vaccine better at protecting against more severe symptoms – but that’s speculative.
With all this in mind, how will the government meet its 2 million a week target, and with which vaccine?
In early December, Health Secretary Matt Hancock said that ‘several million’ doses of the Pfizer vaccine were expected by the end of 2020. That clearly didn’t materialise, and the government won’t now confirm if ‘several million’ Pfizer doses are anticipated, or indeed if we’re going to get any more at all, beyond the million or so already available. Attention now seems to have shifted to the Oxford-AstraZeneca vaccine as the main route to mass vaccination. There seems to be plenty of that available, and much more in production. Initially, supplies will come from the Netherlands, but production will then shift to the UK, for doses to be administered in the UK.
However, there is another problem. Most of the vaccine is only available in bulk containers at the moment, because of a world shortage of ‘fill and finish’ materials, particularly the glass vaccine vials that are used by medical staff administering the vaccine jabs. That’s not a problem for the Pfizer vaccine because US biotech company Pfizer did a deal with US glass manufacturer Corning to secure a supply of vials early on in the development of their vaccine. Sadly, here in the UK, we have no major manufacturers of medical glassware. Of the world’s biggest suppliers, there’s Corning in the US, then two companies in Germany, one in France, and one in Italy. Not unexpectedly, those EU-based companies are targeting their supplies on the EU – so not the UK. The world’s largest manufacturer of medical glassware is Schott, based in Germany. They say they’re reserving their supply of vials for ‘approved vaccines’. While the EU pharmaceutical regulators have now approved the Pfizer and the Moderna vaccines, they have said that approval of the Oxford vaccine is ‘some way off’ and have asked Oxford University and AstraZeneca for further data. The lack of this ability to manufacture medical glassware in the UK has been recognised by the UK government. A manufacturing plant to make vials is now being built in the UK, but won’t be completed till the summer; stable doors and horses come to mind.
The idea of guaranteeing our ability to manufacture both vaccines and the necessary containers and distribution systems should perhaps have been considered rather earlier. Indeed, it should have been considered 40 years ago, when the Thatcher government destroyed the large-scale manufacturing sector in the UK, leaving us with no capacity to make these sort of emergency items, from PPE to refrigeration equipment to glass vials. Fitting perhaps that the new medical glass factory is being constructed in Stoke-on-Trent, once the epicentre of world glass manufacturing and innovation, before the Tories destroyed that city’s manufacturing heritage. Globalism is one thing, but in a global emergency, every nation state will look after its own first.
Importing from China doesn’t seem to be an option, because China doesn’t make medical glassware from the ‘Type 1’ borosilicate glass that comes up to the standard required in this country. Somehow, the problem will be overcome, we hope, even if the government has to pay way over the odds to get hold of the vials, after manufacturers have set aside enough vials for use in the EU for EU approved vaccines. I still feel confident that we’ll muddle through, and we’ll see a significant reduction in cases, and a big reduction on hospital admissions, by the summer. But there are lessons to be learned here. And some of these are lessons that should have been learned decades ago.
